- 05 maart 2020
- Door Prof. dr. ir. Anton A. Kiss
- | 10 min. leestijd
New horizons in reactive distillation
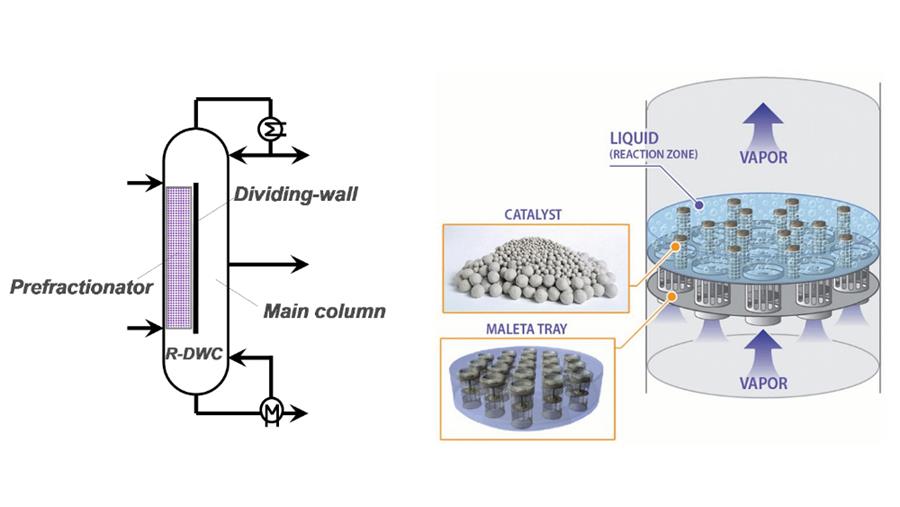
Reactive distillation (RD) reduces capital investment and saves energy because it can get passed equilibrium limitations, simplify complex processes, increase product selectivity and improve separation efficiency. This article gives a brief overview into novel integrated technologies that combine RD principles with new intensified distillation, which leads to new processes and applications.
Sorry, dit is een premium artikel
Dit artikel is exclusief beschikbaar voor abonnees. Abonneer je nu en krijg toegang tot alle artikels.
Ontdek onze abonnementsformules